Adjusted Gross Revenue (AGR) Relief Package for Vodafone Idea
Context: The Union Cabinet approved a restructuring of Vodafone Idea’s AGR dues to ensure sector competition and protect government equity amid the company’s severe financial stress.
More on the News
- The Union Cabinet approved a relief package for Vodafone Idea on December 31, 2025, freezing the company’s adjusted gross revenue dues at ₹87,695 crore as on the same date.
- The frozen dues will be repaid over a ten-year period from FY 2031–32 to FY 2040–41.
- To ensure accuracy in the final payout, the frozen dues will undergo a reassessment by the Department of Telecommunications (DoT) based on the 2020 Deduction Verification Guidelines and audit reports. A government-appointed committee will oversee this process, and its findings will be binding on both parties.
- The decision provides a five-year moratorium on a critical payment of approximately ₹18,000 crore, which was originally due by March 31, 2026.
- Under the new schedule, the dues pertaining to FY 2017-18 and FY 2018-19 will now be payable over a six-year window between FY 2025-26 and FY 2030-31.
- The relief follows Supreme Court observations in October and November 2025, allowing the government to reconsider AGR dues in the public interest.
- The Court highlighted the “larger public interest,” noting the government’s own 49% stake in the telco and the welfare of its 20 crore mobile consumers.
Adjusted Gross Revenue (AGR)
- AGR is the revenue base used by the Indian Department of Telecommunications (DoT) to calculate the license fees and spectrum usage charges (SUC) payable by telecom operators.
- Adjusted Gross Revenue (AGR) in the Indian telecom sector is primarily governed by the Department of Telecommunications (DoT) and overseen by the Telecom Regulatory Authority of India (TRAI).
- Since 1999, India has used a revenue-sharing model where telecom companies pay a percentage (typically 8% for license fees and 3–5% for SUC) of their AGR to the government.
Electronics Component Manufacturing Scheme (ECMS)
Context: Recently, the Ministry of Electronics and Information Technology approved 22 proposals under the third tranche of the Electronics Component Manufacturing Scheme.
More on the News
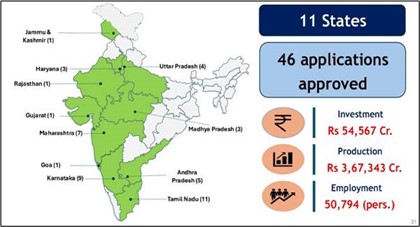
- These approvals involve a projected investment of ₹41,863 crore and a projected production value of ₹2,58,152 crore.
- The approved projects are expected to generate 33,791 direct employment opportunities across the country.
- This tranche follows the earlier approval of 24 applications involving an investment of ₹12,704 crore.
- With this round, a total of 46 applications have been approved under ECMS so far.
- The cumulative approved investment under the scheme now stands at ₹54,567 crore with direct employment for about 51,000 people.
- The approved manufacturing units are spread across eight states, including Andhra Pradesh, Haryana, Karnataka, Madhya Pradesh, Maharashtra, Tamil Nadu, Uttar Pradesh and Rajasthan.
- These approvals include the manufacturing of 11 target segment products, which have cross-sectoral applications such as mobile manufacturing, telecom, consumer electronics, strategic electronics, automotive and IT hardware products. These 11 products are as follows:
- 5 bare components like PCB, Capacitors, Connectors, Enclosures and Li-ion Cell
- 3 sub-assemblies like Camera Module, Display Module and Optical Transceiver
- 3 supply chain items like Aluminium Extrusion, Anode Material and Laminate (Copper Clad)
The Electronics Component Manufacturing Scheme (ECMS)
- It is a flagship initiative of the Government of India, launched on April 8, 2025, to establish a robust domestic ecosystem for high-value electronic components.
- It is managed by the Ministry of Electronics and Information Technology (MeitY), which aims to integrate India into Global Value Chains (GVCs) and reduce import dependency.
National Financial Reporting Authority releases second Audit Practice Toolkit
Context: Recently, the National Financial Reporting Authority (NFRA) published its second Audit Practice Toolkit titled “Risk & Response Memorandum: ROMM (Risk of Material Misstatement) Assessment at Assertion Level for Revenue”.
More on the news
- The toolkit focuses on the assessment of Risk of Material Misstatement at the assertion level, which is a critical phase of the audit process.
- It provides a structured sample document to help auditors identify and respond to risks related to revenue reporting.
- NFRA Chairperson stated that the document is adaptable for audits of different sizes and types.
- The toolkit is expected to serve as a practical guide that practitioners can modify based on facts and circumstances of each audit.
- The initiative continues NFRA’s outreach efforts for improving auditing practices, especially among small and medium firms.
- NFRA has announced plans to issue more Audit Practice Toolkits in other significant audit areas during the remaining financial year.
The National Financial Reporting Authority (NFRA)
- NFRA was constituted on 01st October,2018 by the Government of India under the Sub Section (1) of section 132 of the Companies Act, 2013.
- Its primary functions are aimed at improving the credibility and transparency of financial reporting for Public Interest Entities (PIEs).
- It operates under the Ministry of Corporate Affairs with its headquarters in New Delhi.
9th National Siddha Day
Context: India observes the Siddha Day annually on January 6 to mark the birth anniversary of Sage Agathiyar.
More on the News
- The day will be celebrated in association with the National Institute of Siddha (NIS) and the Central Council for Research in Siddha (CCRS), along with the Directorate of Indian Medicine and Homoeopathy, Government of Tamil Nadu.
- CCRS is the apex body for Siddha research, providing healthcare through 8 peripheral institutes and 5 co-located units registered under the Societies Act in July 2010 under the Ministry of Ayush.
- The theme of the day is “Siddha for Global Health”.
- The Day highlights Siddha medicine’s role in preventive health, research, and global wellness, while raising awareness and reaffirming government efforts to strengthen its place in healthcare delivery, research, and academics.
About Siddha
- Siddha system of medicine is said to have originated from the Shiva cult and the great sage Agasthiyar, also known as Father of Siddha Medicine.
- The Siddha system of medicine derived from the term siddhi (perfection) is an indigenous Indian medical tradition that originated in the Dravidian civilisation.
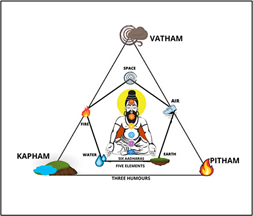
- Siddha medicine is based on the principles of anda pinda thathuvam (the unity of the universe and human body) and 96 thathuvas, encompassing the five elements, three humours, and seven physical constituents that form the physical body (annamaya kosa).
- The three humours (uyir thathu), which govern body functions are:
- Vali (Vatham – air and space),
- Azhal (Pitham – fire), and
- Iyyam (Kapham – water and earth).
- Treatment consists of three distinct categories
- Deva Maruthuvam (Divine method)
- Manida Maruthuvam (Rational method)
- Asura Maruthuvam (Surgical Method)
- “Food is Medicine; Medicine is Food” is one of the core doctrines mentioned in Siddha classics for a healthy life.
Indian Pharmacopoeia 2026 (IP 2026)
Context: Recently, the Ministry of Health and Family Welfare released the 10th edition Indian Pharmacopoeia 2026 (IP 2026) at Dr Ambedkar International Centre, New Delhi.
More on the News
- Indian Pharmacopoeia serves as the official book of standards for drugs in the country and is a cornerstone of India’s regulatory framework for pharmaceuticals.
- The Indian Pharmacopoeia is published by the Indian Pharmacopoeia Commission on behalf of the Ministry of Health and Family Welfare under the Drugs and Cosmetics Act, 1940.
- The 10th edition reflects scientific advancements, global best practices, and India’s growing leadership in pharmaceutical manufacturing and regulation.
- IP 2026 is incorporated with 121 new monographs, now increasing the total number of monographs to 3,340.
- A key feature of IP 2026 is the first-time inclusion of 20 blood component monographsrelated to transfusion medicine, in line with the Drugs and Cosmetics (Second Amendment) Rules, 2020.
- It has strengthened across key therapeutic categories, including anti-tubercular, anti-diabetic and anti-cancer medicines, as well as iron supplements, thereby ensuring more comprehensive standardisation of medicines used under various National Health Programmes.
- India has improved its global ranking in contributions to the WHO pharmacovigilance database from 123rd (2009–2014) to 8th in 2025.
About Indian Pharmacopoeia Commission (IPC)
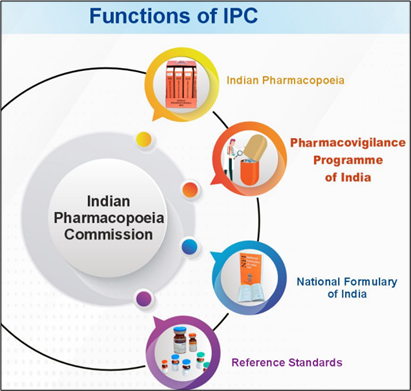
- IPC became operationalised on 1 January 2009 as an autonomous institute.
- It functions under the Ministry of Health and Family Welfare.
- The Secretary, Ministry of Health and Family Welfare, is the Chairperson of IPC, and the Secretary-cum-Scientific Director acts as the Chief Scientific and Executive Officer.
- The Indian Pharmacopoeia defines standards for the identity, purity, and strength of drugs manufactured, imported, sold, or distributed in India.
- IPC’s mandate includes revising and publishing the IP and the National Formulary of India (NFI), supplying IP Reference Substances, and providing training on pharmacopoeial matters.
Peru granted legal rights to Amazonian stingless bees
Context: Recently, Peru has passed an ordinance, which granted legal rights to Amazonian stingless bees, making them the first insects in the world to get legal rights.
More on the News
- The declaration was developed with local leaders and community members, recognising that indigenous rights, culture, and spiritual beliefs are deeply connected to the well-being of stingless bees.
- The ordinances officially recognise the bees’ inherent right to exist, thrive, and be protected as follows:
- Right to Exist and Flourish: recognises their intrinsic right to live and maintain their presence in the ecosystem.
- Healthy Habitat and Environment: Right to live in an environment free from pollution, including specific legal protections against pesticides and the burning of hives.
- Regenerate Natural Cycles: Protection for their life cycles, including nesting and foraging, to ensure they can maintain healthy populations.
- Ecological Stability: Right to stable climatic conditions, aimed at addressing threats from climate change.
- Legal Representation: The bees can now be legally represented in court if their rights are threatened by harm or habitat destruction.
About Stingless bees
- Stingless bees are bees with no stingers or harmless ones, found mainly in tropical regions.
- About half of the 500 known species live in the Amazon, with over 170 species in Peru.
- They have sustained tropical forests for nearly 80 million years and pollinate over 80% of Amazonian flora, including crops like cacao, coffee, and avocados.
- Asháninka people also use stingless bee honey as medicine, which studies show has anti-inflammatory, antibacterial, and antiviral properties and is distinct in taste and texture from commercial honey.

