SYLLABUS
GS-2: Issues relating to the development and management of Social Sector/Services relating to Health.
Context:
Recently, the Indian Council of Medical Research (ICMR) has invited Expression of Interest (EoI) from eligible organisations, companies, and manufacturers for the development and production of monoclonal antibodies (mAbs) against Nipah viral disease.
More on the News
- This initiative aims to create an indigenous and deployable stock of monoclonal antibodies as the only viable biomedical countermeasure in the absence of a licensed vaccine.
- The ICMR will extend technical support and expert guidance to industry partners throughout the research, development, and production phases.
- The monoclonal antibodies will be used both for treatment in early infection and for post-exposure prophylaxis in high-risk contacts.
- The National Institute of Virology in Pune has already made significant progress in experimental research related to this therapy.
- The repeated Nipah outbreaks in India, especially in Kerala since 2018, have highlighted the urgency of developing indigenous therapeutic solutions.
- The ICMR stated that the project will strengthen national health security and reduce dependency on foreign sources during future outbreaks.
Indian Council of Medical Research (ICMR)
ICMR was established in 1911 is one of the oldest medical research bodies in the world.
Mandate:
- Apex body in India for the formulation, coordination, and promotion of biomedical research.
- Conduct, coordinate, and implement medical research for societal benefit.
- Translate medical innovations into products/processes and introduce them into the public health system.
Vision: Translating Research into Action for Improving the Health of the Population
Headquartered in New Delhi, India.
About the Monoclonal Antibodies (mAbs)
- Monoclonal antibodies (also called moAbs or mAbs) are proteins made in laboratories that act like natural antibodies produced in our bodies.
- Antibodies are parts of the immune system that seek out antigens (foreign materials) and attach to them to neutralise or destroy them.
- The word “monoclonal” refers to the fact that these laboratory-created antibodies are clones, exact copies of a single type of antibody.
- They provide passive immunity by directly targeting pathogens, helping the body fight infections.
Importance for Nipah
ICMR emphasised that monoclonal antibody stocks are the only currently feasible biomedical countermeasure for Nipah, given the high fatality and absence of vaccines.
mAbs can serve as post-exposure prophylaxis for high-risk groups such as:
- Healthcare workers exposed without adequate protection,
- Family members in close contact with patients,
- Laboratory personnel with accidental exposure.
If administered early, mAbs can prevent disease onset, as demonstrated in animal models.
In early-stage patients, monoclonal antibodies may:
- Reduce viral load,
- Limit disease progression,
- Complement supportive critical care.
This dual use, preventive and therapeutic, makes mAbs a vital tool in outbreak response.
About Nipah Virus
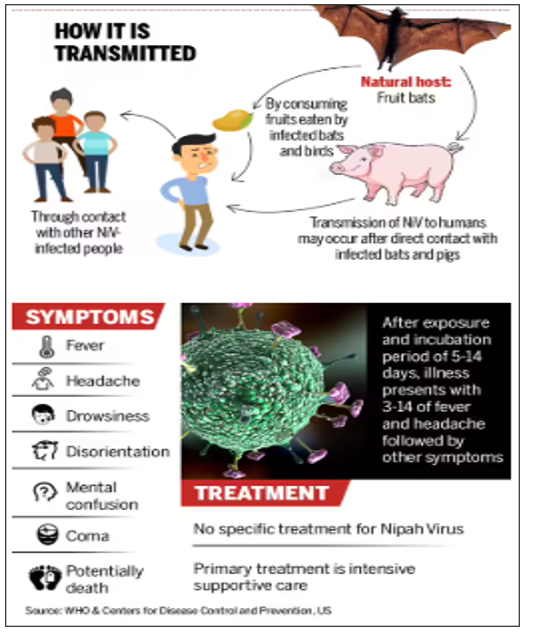
- Nipah virus (NiV) is a zoonotic virus (it is transmitted from animals to humans) and can also be transmitted through contaminated food or directly between people.
- It was first recognised during an outbreak in Malaysia in 1998, which was linked to infected pigs.
- In India, most infections have been associated with the consumption of fruits or palm sap contaminated by fruit bat secretions.
- The infection can be asymptomatic or cause severe respiratory illness and fatal encephalitis in humans.
- The incubation period usually ranges from 4 to 14 days but may extend up to 45 days in some cases.
- There is currently no approved antiviral drug or vaccine for Nipah virus infection.
- Fruit bats of the Pteropus genus are the natural reservoir, and the infection can also cause severe disease in pigs.


