Syllabus:
GS3: Achievements of Indians in Science & Technology; Indigenization of Technology and Developing New Technology.
Context:
Recently, a Petition in the Kerala High Court asked Govt to invoke a compulsory licensing clause that grants someone other than the patent holder the right to make a medicine.
More on the News
- The petitioner has been surviving with the help of a ₹50 lakh government grant provided under the National Policy for Rare Diseases.
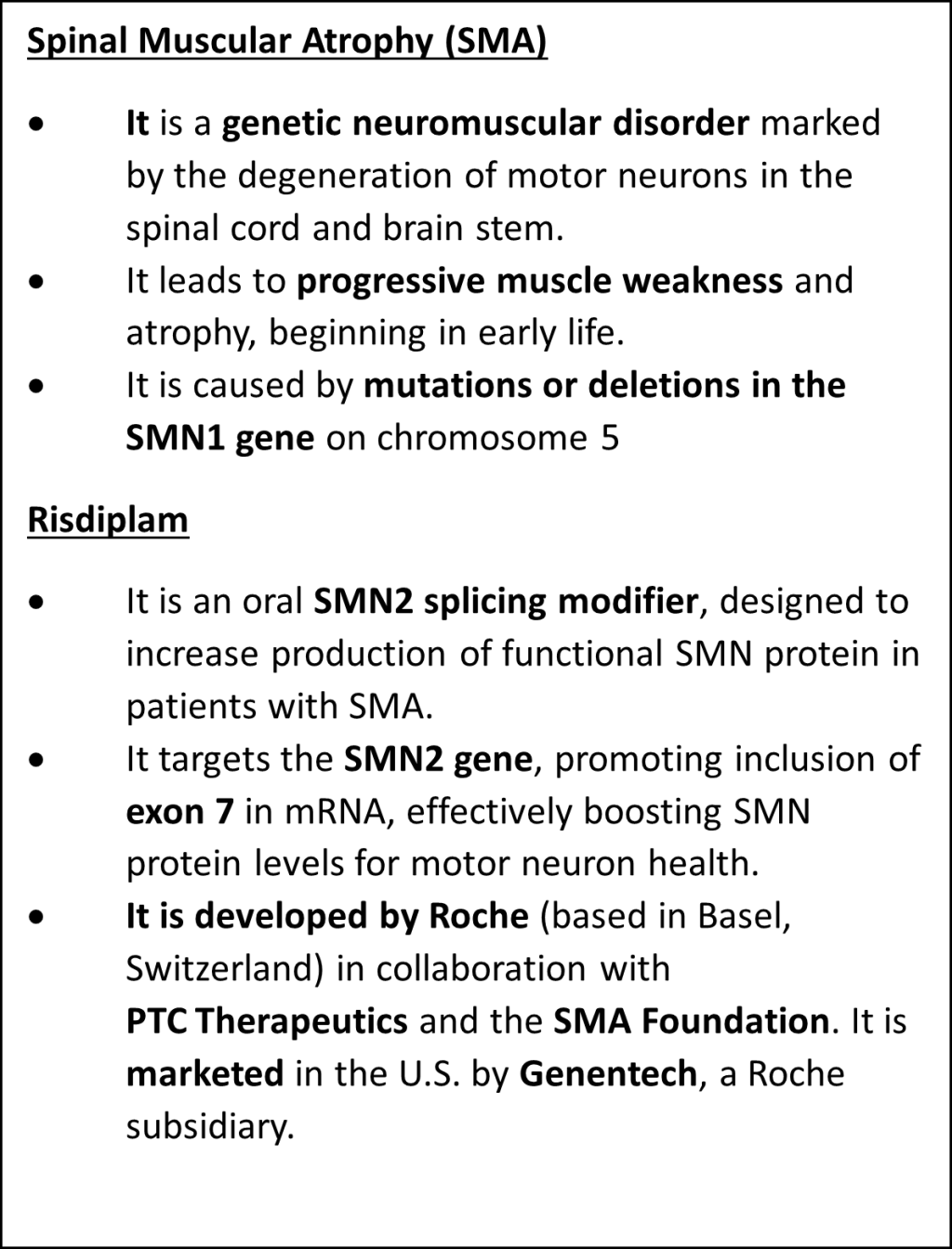
- She is fighting Spinal Muscular Atrophy, a rare disease. Now, she is campaigning to make life-saving patented medicines more affordable for people suffering from rare diseases.
- To make the costly Spinal Muscular Atrophy medicine affordable, a person filed a petition in the Kerala High Court in December 2023, urging the government to invoke compulsory licensing under Section 84 of the Indian Patents Act, 1970.
- Risdiplam is the first oral treatment approved for Spinal Muscular Atrophy (SMA) and is available for use in adults, children, and infants from birth.
Compulsory Licensing

- Compulsory licensing is when a government allows someone else to produce a patented product or process without the consent of the patent owner or plans to use the patent-protected invention itself.
- It is one of the flexibilities in the field of patent protection included in the WTO’s agreement on intellectual property, the TRIPS (Trade-Related Aspects of Intellectual Property Rights) Agreement.
- Compulsory licensing is a mechanism available under the Trade-Related Aspects of Intellectual Property Rights (TRIPS) Agreement.
- In 2012, Natco Pharma, a New Delhi-based company, obtained a compulsory license to manufacture Nexavar, a cancer drug originally patented by German multinational Bayer. This landmark decision allowed Natco to produce a generic version of Nexavar, making it significantly more affordable for patients in India.
Key Provisions Related to Compulsory Licensing
- Section 84 of the Indian Patents Act, 1970: It allows any person interested to apply for a compulsory license on a patent after three years from the date of its grant, under specific circumstances.
- Section 92 of the Indian Patents Act, 1970: It allows acts done in good faith for someone’s benefit without their consent when the person can’t consent or time doesn’t permit, provided the intent is genuinely to help.
- Section 100 of the Indian Patents Act, 1970: It allows the Central Government or its authorized agents to use a patented invention for governmental purposes, even without the patentee’s consent or royalty, under certain conditions.
Significance of the Compulsory Licensing
- It allows for the production of generic versions of patented drugs, making them more affordable and accessible to the general population.
- Compulsory licensing acts as a safeguard against potential abuse of patent rights, such as excessive pricing or non-working of patents
- It can encourage domestic companies in developing countries to invest in research and development, rather than relying solely on importing patented products.
- Compulsory licensing can be granted when the reasonable expectations of the public with respect to the patented invention have not been met, ensuring access to the invention.
Challenges of the Compulsory Licensing
- Granting compulsory licenses can diminish the potential economic rewards for companies investing in research and development (R&D).
- Compulsory licensing can erode the competitive advantage gained from innovation, as others can produce the same product without bearing the initial R&D costs.
- Compulsory licensing can discourage technology transfer agreements, joint ventures, and other forms of collaboration between companies in developed and developing countries.
- Some developing countries may lack the technical and legal capacity to effectively manage compulsory licensing processes.
Mains Practise question
Critically examine the role of compulsory licensing in ensuring access to life-saving medicines for rare diseases. Support your answer with recent developments and suggest policy measures to address affordability and innovation. (15M, 250W)