Context:
Recently, researchers at the Christian Medical College, Vellore have successfully applied gene therapy to treat severe haemophilia A.
More on the News

- The results of the study were reported in the New England Journal of Medicine (NEJM).
- The trial was held at Christian Medical College, Vellore, and financially supported by the Union Department of Biotechnology.
About Haemophilia
It is a rare disorder in which the blood doesn’t clot in the typical way because it doesn’t have enough blood-clotting proteins (clotting factors).
The 3 main forms of haemophilia include:

- Hemophilia A: This is caused by a lack of or low levels of the blood clotting factor VIII. It is the most common type (Classic) of haemophilia.
- Hemophilia B: This is caused by a deficiency of or low levels of blood clotting factor IX. This is also called Christmas disease or factor IX deficiency.
- Hemophilia C: Some healthcare providers use this term to refer to a lack of clotting factor XI. It is a rare condition.
The primary approach to treating haemophilia is called replacement therapy. This treatment regimen involves injecting into a vein, concentrates of ‘clotting factor’ to prevent bleeding.
The main challenge with clotting factors is that the body’s own antibodies can destroy the clotting factor before it has a chance to work, thus defeating the whole idea of replacement therapy.
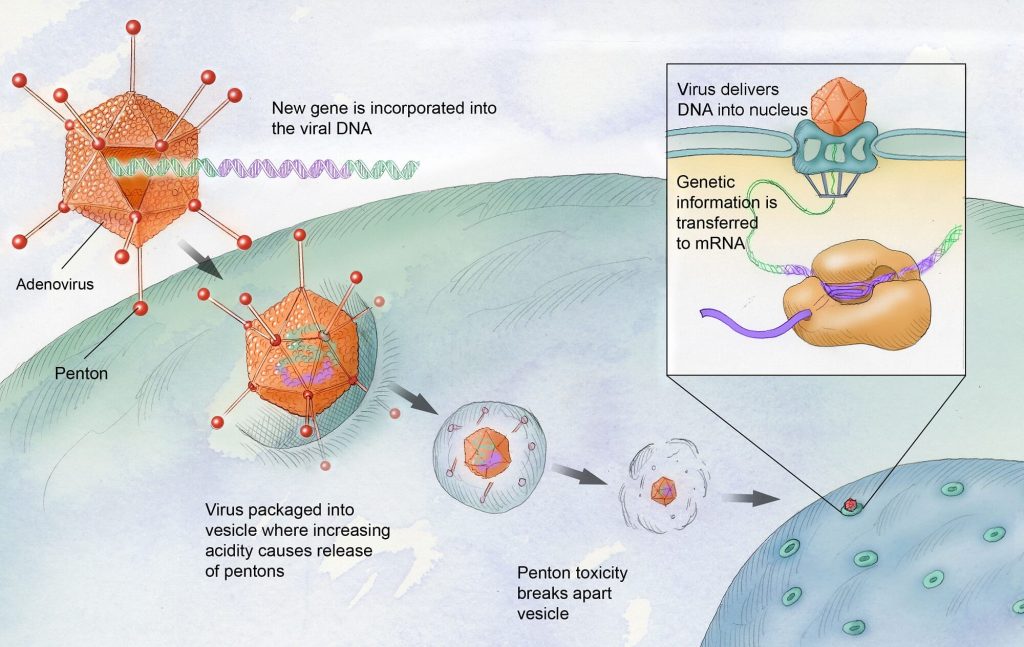
Gene Therapy in Haemophilia
This therapy uses a lentivirus as a vector instead of an adenovirus.
Adenovirus infections are common subsequently many people have antibodies against it, which could reduce the effectiveness of treatments like Roctavian (Adenovirus-based gene therapy).
Lentivirus infections are less common so fewer people are likely to have antibodies thus making the treatment more effective.
This method involves transferring genes into adult stem cells using the lentiviral vector, which integrates with the body’s cells.
- This is different from in vivo transfer to liver cells using a non-integrating AAV vector.
The lentivirus-based approach is expected to provide reliable, life-long production of the clotting factor without side effects.
Significance of the Study
- The approach using lentivirus is considered safer than adenovirus-based gene therapies.
- The method may make gene therapy treatments accessible to children, as it is safer and more effective.
- The study demonstrates that advanced gene therapy research can be conducted even in resource-limited environments like India.
- The potential to localize the manufacturing of gene therapies in India could reduce treatment costs and improve access to gene therapy both within and outside India.
- This breakthrough could address challenges like liver health and maturity, which are critical for successful treatment, and eliminate the need for immunosuppressive therapy.
Haemophilia in India
- A prevalence of haemophilia A of 4 per 1,00,000, the estimated number of haemophilia patients in India would be around 50 thousand. Thus, India may have over 70,000 patients with haemophilia A and B.
- According to the Haemophilia and Health Collective of North (HHCN), India has the second-largest population of haemophilia cases in the world.